Kobayashi Group
Please click here for new Web site of Kobayashi laboratory
Current study
Living organisms contain a myriad of organic molecules. In our laboratory we are interested in making naturally occurring bioactive molecules (natural products) derived from plants, mushrooms, seaweeds, and other organisms. Such natural products often act as potential seeds for new medicines, agrochemicals, cosmetics, flavorings, and nutritional supplements. Our general philosophy to making a natural product is to adopt an "eco-friendly synthesis", in which we endeavor to reduce the number of synthetic steps, cumbersome purifications, harmful solvents, undesirable formation of byproducts, and waste materials.
The current research topics are as follows.
- Divergent total synthesis and structure-activity relationship of bioactive components derived from medicinal mushrooms
- Synthesis and determination of the absolute structures of marine-derived brominated acetogenins through stereospecific transformations
- Concise syntheses of structurally novel drug lead molecules starting from structurally complex abundant natural products
- Development of green synthetic reactions and methods
☆From recent publications
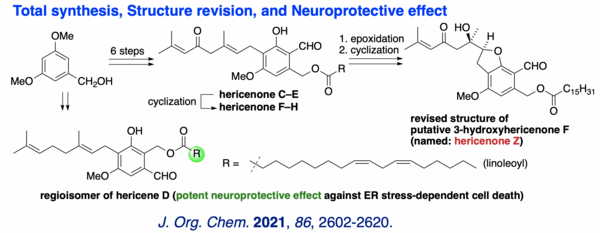
(12) Total Synthesis, Structure Revision and Neuroprotective Effect of Hericenones C-H and Their Derivatives
The first total syntheses of hericenones C-H and "putative 3-hydroxyhericenone F" were achieved. Highlights of the synthesis include the straightforward construction of the resorcinol core and the geranyl side chain, assembly of the natural product skeleton by sequential O-geranylation and a clay/zeolite-mediated O→C rearrangement reaction, as well as a biomimetic cyclization to form a variety of bicyclic natural hericenones and their congeners. The structure of the "putative 3-hydroxyhericenone F" was revised as the 5-exo cyclization product (named: hericenone Z) of epoxyhericenone C through in-depth analyses of the cyclization modes in addition to NMR spectroscopic studies. To gain insights into the biological functions of geranyl-resorcinols in H. erinaceus, potential neuroprotective effects against endoplasmic reticulum (ER) stress-dependent cell death were evaluated systematically to clarify a fundamental structure-activity relationship. Among the compounds assayed, the linoleate-containing hericenone analog, i.e. the regioisomer of hericene D, was found to possess the most potent neuroprotective effect against tunicamycin and thapsigargin-induced ER stress-dependent cell death.
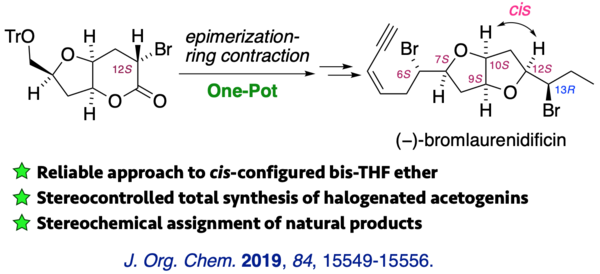
(11) Stereocontrolled Total Synthesis of (+)-Isolaurenidificin and (−)-Bromlaurenidificin
The first total syntheses of (+)-isolaurenidificin and (−)-bromlaurenidificin, the latest acetogenins of the 2,6-dioxabicyclo[3.3.0]octane class, were reported. The synthesis features a completely stereoselective one-pot epimerization-ring contraction to establish the cis configuration with respect to C10-H and C12-H of the tetrahydrofuran ring. Six stereogenic centers and an olefin geometry were constructed in a highly stereoselective manner. Absolute configurations of the natural products were deduced by the comparison of NMR data and specific rotations.
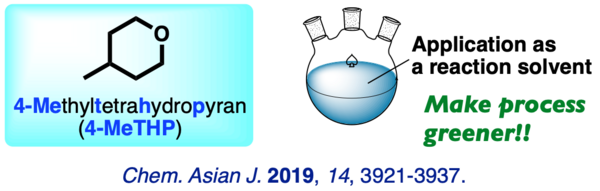
(10) 4-Methyltetrahydropyran (4-MeTHP): Application as an Organic Reaction Solvent
A comprehensive study on the performance of 4-methyltetrahydropyran (4-MeTHP) as a reaction solvent is described. The broad applications and higher stability under free-radical conditions make this solvent a promising alternative to common organic solvents. This study provides key data for process chemists in choosing a reaction solvent under green chemistry perspectives.
(9) Total syntheses and endoplasmic reticulum stress suppressive activities of hericenes A−C and their derivatives
We report the syntheses and neuroprotective activities of hericenes and their derivatives against endoplasmic reticulum (ER) stress-dependent cell death. Four natural products, including hericenes A−C and hericenol A, and five synthetic derivatives were synthesized and their protective activities were evaluated. In designing the synthetic derivatives, we focused on the binding position of the fatty chain. Hericenes B and C showed moderate protective activity against thapsigargin-induced ER stress-dependent cell death. In contrast, their regioisomers (with respect to the position of the fatty chain) exhibited higher protective activity against tunicamycin-induced ER stress. This study clearly shows that the number and the binding position of the fatty chain are critical for protective activity against ER stress-dependent cell death.
We report short syntheses of (−)-tripterifordin and (−)-neotripterifordin, potent inhibitors of HIV replication, from stevioside, a natural sweetener used worldwide. The key transformations are reduction at C13 through the formation of a tertiary chloride and subsequent three-step lactonization including a selective iodination at C20 by the photoreaction of the C19-alcohol. The title compounds were reliably obtained from stevioside in 9 and 11 steps (with 5−7 isolation steps), respectively. Additionally, the related lactone-containing ent-kaurenes, doianoterpenes A and B, and two more natural products were synthesized.
Grignard reactions using cyclopentyl methyl ether as a reaction solvent were systematically investigated. Using the examples of tramadol and tamoxifen, recycling of the solvent system made the synthetic processes of pharmaceutical products more environmentally friendly.
We have reported a highly stereocontrolled total synthesis of one of the possible stereoisomers of laurenidificin. While the synthetic compound was not identical to the natural product, the absolute stereochemistry of the natural product was proposed on the basis of NMR analyses. Moreover, a formal total synthesis of (+)-aplysiallene was achieved by extending the ring contraction strategy.

(5) Divergent Synthesis of Bioactive Resorcinols Isolated from the Fruiting Bodies of Hericium erinaceum: Total Syntheses of Hericenones A, B, and I, Hericenols B-D, and Erinacerins A and B
Total syntheses of 5'- and 7'-oxidized geranyl resorcylates isolated from the fruiting bodies of Hericium erinaceum and the submerged cultures of a Stereum species were achieved. The synthesis features derivatization of a suitably functionalized 5'-oxidized geranyl phthalide into a series of natural products by divergent functional group manipulations. Eight natural products including hericenones A, B, and I, hericenols B-D, and erinacerins A and B were synthesized (hericenol B and erinacerin B were synthesized as racemates). The structure of hericenone B was revised as the carbonyl regioisomer of the original assignment. In addition, this study indicated that hericenols C and D were artifacts resulting from degradation of hericenol B.
(4) Evaluation of cyclopentyl methyl ether (CPME) as a solvent for radical reactions
Cyclopentyl methyl ether (CPME), developed by Zeon Corporation, emerged as an environmentally-benign ethereal solvent, due to recyclability, low toxicity, safety and so on. However, its use in organic syntheses has not been developed so much. We have shown that CPME was an effective solvent for radical additions, reductions, and radical-containing one-pot reactions. Furthermore, degradation pathway of CPME under radical conditions was elucidated.
(3) Total synthesis and structural revision of hericerin
Hericerin, isolated from the mushroom Hericium erinaceum, shows inhibitory activity against pine pollen germination and tea pollen growth, and therefore is expected to be useful as a research tool for agrochemical discovery. We achieved the first total synthesis of hericerin starting from the originally prepared phthalide intermediate. Our findings reveal that the actual structure of hericerin is the carbonyl regioisomer of the original compound.

(2) Stereocontrolled synthesis of substituted bicyclic ethers through oxy-Favorskii rearrangement: Total synthesis of communiol E
We developed a convenient, stereocontrolled route to branched cis-fused bicyclic ethers through halolactonization, α-bromination, and oxy-Favorskii rearrangement. The total synthesis of communiol E, a bicyclic polyketide isolated from Podospora communis, was achieved based on this method.

(1) Total synthesis of hericene A featuring a CuBr2-mediated one-pot multi-step reaction
Hericenone family isolated from mushroom Hericium erinaceum shows diverse biological activities. We have investigated a practical route to 6-bromo-5,7-dihydroxyphthalide 5-methyl ether, a versatile intermediate in the synthesis of hericenones and related bioactive polyphenols. The synthesis features a combination of tandem Michael addition-Claisen condensation and CuBr2-mediated multi-step reactions. With this product in hand, total synthesis of hericene A (5'-deoxohericenone C), a leading polyphenol of this family, was achieved.